In recent years, reports of fires and even explosions caused by lithium-ion batteries are not uncommon.
Let's learn about the thermal stability and overcharge, high temperature and short circuit safety analysis of
lithium batteries together:
Lithium-ion batteries are mainly composed of negative electrode material, electrolyte and positive electrode
material. The chemical activity of the negative electrode material graphite is close to that of metallic lithium in
the charged state. The SEI film on the surface decomposes at high temperatures, and the lithium ions embedded
in the graphite react with the electrolyte and the binder polyvinylidene fluoride to release a lot of heat.
Alkyl carbonate organic solutions are commonly used as electrolytes, which are flammable. The positive electrode
material is usually a transition metal oxide, which has a strong oxidizing property in the charged state, and is easily
decomposed to release oxygen at high temperature. The released oxygen undergoes an oxidation reaction with the
electrolyte, and then releases a large amount of heat.
Therefore, from the point of view of materials, lithium-ion batteries have a strong risk, especially in the case of
abuse, safety issues are more prominent.
1. Thermal stability analysis of lithium-ion battery materials
The fire hazard of lithium-ion batteries is mainly determined by the amount of heat generated by chemical reactions
in various parts of the battery. In the final analysis, the fire hazard of lithium-ion batteries depends on the thermal
stability of the battery material, and the thermal stability of the battery material depends on the chemical reaction
between its internal parts. At present, people mainly use Differential Scanning Calorimeter (DSC), Thermogravimetric
Analyzer (TGA), Adiabatic Accelerating Calorimeter (ARC), etc. to study the thermal stability of battery-related materials.
1 Influencing factors of the thermal stability of anode materials:
The onset temperature of the negative electrode material's heat generation increases with the increase of the
particle size.
The thermal stability of lithium-intercalated natural graphite with different particle sizes was studied by DSC. It was
found that all samples showed 3 exothermic peaks. The first exothermic peak of the sample is located near 150℃,
and the positions of the latter two exothermic peaks are obviously different. The onset temperature of the latter two
exothermic peaks increases with the increase of particle size. The study shows that the first exothermic peak is the
decomposition of SEI film, and the latter two exothermic peaks are the reaction of lithium intercalation graphite with
PVDF and electrolyte.
Using ARC to study the relationship between the specific surface area of graphite materials and thermal stability, it
was found that when the specific surface area of graphite materials increased from 0.4 m2/g to 9.2 m2/g, the
reaction rate increased by two orders of magnitude. Therefore, the reaction rate of the carbon anode material
increases with the increase of the specific surface area.
Carbon materials with different structures produce different amounts of heat. Graphite structures produce more
heat than amorphous carbon structures.
The thermal stability of carbon fiber, hard carbon, soft carbon and MCMB four different structure carbon materials
was studied by DSC. The study found that the first exothermic peak of the four types of carbon all appeared at
100°C. This exothermic peak is considered to be caused by the decomposition of the SEI film; as the temperature
rises to 230°C, the carbon structure and specific surface area are thermally stable to the material The influence of
the nature is gradually emerging, and the carbon electrode materials with graphite structure (carbon fiber, MCMB)
generate more heat than the carbon electrode materials with amorphous structure (soft carbon, hard carbon). XRD
shows that at about 230°C, the total amount of lithium intercalation loss is linearly related to the carbon specific
surface area.
2 Factors affecting the thermal stability of cathode materials:
The initial temperature of the reaction between the positive electrode material and the electrolyte increases as the
stoichiometric number decreases.
The effect of the change of x on the reaction of LixCoO2, LixNiO2, LixMn2O4 and LixC6 with electrolyte was studied
by DSC. It is concluded through research that there is a widespread exothermic reaction between the electrolyte and
the cathode material. When the value of x decreases, the reaction temperature rises to the range of 200-230℃, and
the LixCoO2, LixNiO2, and LixMn2O4 materials all react strongly with the electrolyte. .
The thermal stability of LixCoO2 was studied with ARC. Above the critical temperature, LixCoO2 undergoes an
oxygen release reaction and releases a lot of heat. When x=0.25, the onset temperature of the exothermic reaction
is approximately 230°C. Li Yi et al. measured the natural reaction temperature of 18650 LiCoO2 in the heat resistance
test to be 170°C, indicating that the onset temperature of the decomposition reaction is lower. Therefore, it can be
seen that the onset temperature of the decomposition reaction of the positive electrode material increases with the
decrease of x.
The higher the content of Ni in the cathode material, the more unstable it is, and the higher the content of Mn, the
more stable it is.
The thermal stability of Li1-xNi1-2xCoxMnxO2 materials with different components was studied by DSC. It was
found that with the decrease of Ni content, the exothermic onset temperature and peak temperature of
Li1-xNi1-2xCoxMnxO2 were higher, and the heat production was less. Maeneil et al. studied the exothermic heat
of the reaction of several cathode materials with 1mol LiPF6 EC/DEC, as shown in Table 1.
Table 1 Thermal stability of common cathode materials
3 Influencing factors of electrolyte thermal stability:
The organic solvent DMC is an important factor causing the instability of the electrolyte, and the higher the DMC
content, the more unstable the electrolyte.
The electrolyte solution of EC+DEC, EC+DMC, PC+DEC and PC+DMC mixed solvent with 1mol/L LiPF6 dissolved
in a closed container was studied by DSC, and it was found that the electrolyte solution containing DMC was better
than the electrolyte solution containing DEC. More likely to react.
The electrolyte can make the positive electrode react at a lower temperature, and different solvents and lithium
salts in the electrolyte are suitable for different positive electrode materials.
The exothermic reaction between Li0.5CoO2, LiMn2O4 charged positive electrode and electrolyte was studied by
ARC and XRD methods, respectively. Studies have shown that the decomposition reaction of Li0.5CoO2 powder
occurs when the temperature is greater than 200°C, and oxygen is released, and the exothermic reaction with
EC/DEC solvent occurs at 130°C. After LiPF6 is added to the solvent, the reaction is inhibited. For the LiMn2O4
material, the crystal form transition occurs at 160°C and exothermic, and the presence of solvent has no effect
on this reaction. After adding LiPF6 to the electrolyte, as the concentration of LiPF6 increases, the reaction between
LiMn2O4 and the electrolyte intensifies.
2. Safety analysis of lithium-ion battery abuse
The safety of lithium-ion batteries mainly depends on the thermal stability of battery materials, and is also closely
related to abuse conditions such as battery overcharge, needle stick, extrusion, and high temperature.
1 Overcharge safety analysis:
The overcharge test simulates the potential safety hazards of the battery when there is an error in the charger
voltage detection, the charger fails or the wrong charger is used.
The thermal runaway caused by overcharge may come from two aspects: on the one hand, the Joule heat generated
by the current, and on the other hand, the reaction heat generated by the side reactions of the positive and negative
electrodes. When the battery is overcharged, the voltage of the negative electrode gradually increases. When the
amount of delithiation of the negative electrode is too large, the delithiation process becomes more and more
difficult. This causes the internal resistance of the battery to increase sharply, and therefore generates a large
amount of Joule heat. It is more obvious when charging at a rate. The overcharged high-voltage positive electrode
oxidant emits a lot of heat, and the negative electrode will also react with the electrolyte exothermic when the
temperature rises. When the heat release rate is greater than the heat dissipation rate of the battery, and the
temperature rises to a certain level, thermal runaway will occur.
Tobishim et al. comparatively studied the overcharge performance of aluminum shell prismatic batteries using
LiCoO2 and LiMn2O4 as the positive electrode materials. The results show that LiCoO2 cells will explode when
charged with a current of 2C to a voltage of 10V, while LiMn2O4 cells are charged with 2C. /10V and 3C/10V did
not smoke, catch fire or explode during overcharging, but only swelled, indicating that Mn has better resistance to
overcharging than Co. Leising et al. studied the influence of different graphite ratios on the overcharge performance
of LiCoO2 cells, and the results showed that the overcharge performance of the cells mainly depends on the positive
electrode material and does not change with the increase of the amount of graphite. This shows that the precipitation
of metallic lithium in the negative electrode during the overcharge process is not the key to the overcharge
performance, but the thermal stability of the excessively delithiated LiCoO2 or the oxidation reaction of the
electrolyte on its surface.
2 High temperature safety analysis:
The simulated environment high temperature test can be carried out by using the hot box test. The hot box test is to
simulate the improper use of the battery at a high temperature, such as placing a mobile phone in an exposed car, or
putting a mobile phone or electronic product in a microwave oven, the temperature can reach 130 ℃ or even 150 ℃.
In the case of thermal abuse, the heat source is not only from the positive and negative electrode materials inside the
battery and its reaction with the electrolyte, the separator melts and shrinks at high temperatures, which causes the
positive and negative electrodes to short circuit. The Joule heat generated by the short circuit is also an important
heat source during the hot box test. . Table 2 summarizes the thermal behavior of the lithium-ion battery system
within a certain temperature range for the electrolyte system of 1mol/L LiPF6/(PC+EC+DMC).
Table 2 The main thermal behavior in the lithium-ion battery system

When the temperature is between 90°C and 120°C, the meta-stable layer of the solid electrolyte interface membrane
(SEI) formed on the surface of the carbon negative electrode after multiple charges and discharges first generates
heat of decomposition; as the temperature rises, the separator melts by endothermic heat; When the temperature is
180~500℃, the positive electrode and the electrolyte have a strong exothermic reaction and produce gas; the SEI
film can prevent the interaction between the lithium intercalation carbon and the organic electrolyte. When the
temperature is higher than 120℃, the SEI film will not protect the negative electrode if it breaks. , The negative
electrode material may start to react with the solvent exothermicly and generate gas. When the temperature rises
to 240~350℃, the fluorine-containing binder starts to react with the lithium-intercalated carbon violently, releasing
a lot of heat, and the negative electrode reacts with the electrolyte. Lithium may be exhausted, so this reaction will
not occur; if the temperature continues to rise to 660°C, the Al current collector will undergo endothermic melting.
These conditions are very dangerous for large lithium-ion power batteries and affect the battery life and safety.
3 Short circuit safety analysis:
The short circuit of the battery is divided into external short circuit and internal short circuit. External short circuit
generally refers to the short circuit caused by direct contact between the positive and negative electrodes; internal
short circuit refers to the short circuit in the area of the battery that is affected by foreign objects when the battery
is pierced by a sharp object or is impacted or squeezed.
Safety analysis of external short circuit
The external short-circuit safety research is tested by the method of connecting the positive and negative poles
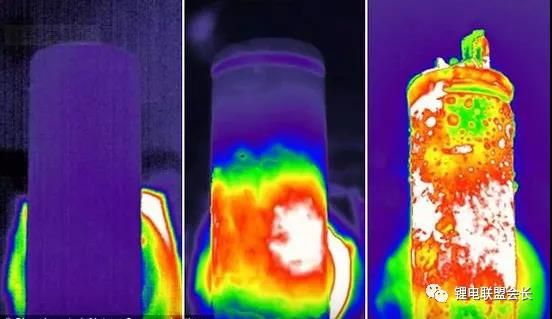
directly externally through wires. Li Yi and others conducted a study on the external short circuit of the battery.
They studied the 18650 lithium cobalt oxide lithium-ion batteries and 6-cell notebook batteries (6 18650 batteries,
3 in series for 1 group, 2 groups in parallel, removing the protection circuit) The positive and negative electrodes
are short-circuited with wires, and a thermocouple is attached to the surface of the battery to detect the
temperature change of the battery surface. Use a paperless recorder to record the temperature curve of the battery
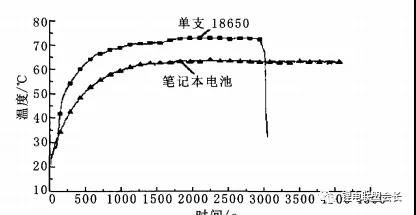
surface. The temperature curves of the two sets of tests are shown in Figure 1.

It can be seen from Figure 1 that the highest temperatures of the two sets of batteries are 73.3°C and 65.1°C,
respectively. Although such temperatures will not cause the battery to burn and explode, because of its
continuous heat release, for a large-capacitance battery pack, If the heat cannot be dissipated in time, it may
cause a fire or even an explosion.
Safety analysis of internal short circuit
The safety research on the internal short circuit of the battery generally uses methods such as acupuncture and
squeeze to test, the purpose is to simulate the situation of the battery being punctured, collided, squeezed and
so on by foreign objects. Acupuncture causes a short circuit of the battery at the acupuncture point. The short-circuit
area forms a local hot zone due to a large amount of Joule heat. When the temperature of the hot zone exceeds the
critical point, it will cause thermal runaway, causing smoke, fire or even explosion hazards. Extrusion is similar to
acupuncture, both of which cause local internal short circuits and may cause thermal runaway. The difference is that
extrusion does not necessarily cause damage to the battery casing. If the casing is not damaged, it means that the
flammable electrolyte will not leak from the hot zone, and the heat dissipation effect from the hot zone is worse.
It is often much more difficult to test the local internal short circuit of the battery caused by squeezing and needle
sticking than the external short circuit test of the battery. This is because when the battery is externally
short-circuited, the internal heat of the battery is often evenly released, and the Joule heat generated by the external
short-circuit battery will not Directly trigger the thermal runaway reaction of the battery.
Test conditions such as acupuncture and extrusion have a greater impact on the test results. This is because the
internal short-circuit conditions caused by the acupuncture and extrusion tests under different conditions are
different. The size of the internal short-circuit resistance has a greater impact on the heat generation power in
the short-circuit area. Impact. There are 4 forms of internal short circuit in the battery: (1) between Al current
collector and negative electrode material (LiC6, C6); (2) between Al current collector and Cu current collector; (3)
between positive electrode material and LiC6; 4) Between the positive electrode material and the Cu current
collector.
Santhanagopalan et al. established a battery electrochemical finite element thermal model to systematically simulate
and analyze the internal heat release power and battery temperature of the battery under these four short-circuit
situations, and designed corresponding experiments to verify it. The results show that the short circuit between the
Al current collector and the charged graphite is the most dangerous, because in this case the short circuit resistance
is small, the current is large, the thermal power is high, the heat conduction and heat dissipation are relatively slow,
and the carbon anode has high activity, so it is easy This caused a series of subsequent electrical and chemical
reactions, leading to accidents.
3. Conclusion
Through thermal stability analysis of lithium-ion battery anode materials, cathode materials and electrolyte, the
main factors affecting the thermal stability of lithium-ion batteries are summarized, and the fire hazard of lithium-ion
batteries during overcharge, external high temperature and short circuit abuse A detailed analysis of the characteristics
provides a reference for the safe use of lithium-ion batteries. When more people pay attention to the material
dangers of lithium-ion batteries, and at the same time strengthen the safety management of the production, storage
and use of lithium-ion electronics, lithium-ion battery fires will be greatly reduced.